Base editing: What is it and what does it mean for healthcare?
This week, we take an in-depth look at the genome-editing technology that has been making headlines worldwide
Base editing has been around since 2016; however, as we reported last month, it has recently made headlines for curing a child’s leukaemia as part of a ground-breaking new cell therapy. So what exactly is base editing, and what potential does it have to improve treatment in other areas of healthcare?
Is base editing the same as CRISPR?
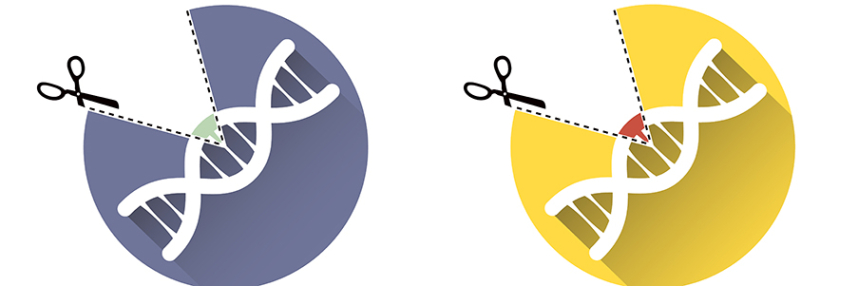
Base editing is a type of genome editing – a way to make targeted changes to the sequence of a piece of DNA.
CRISPR, in common parlance, refers to genome editing approaches that use a CRISPR guide RNA molecule to find their target. More accurately, CRISPR is an acronym that stands for clustered regularly interspaced short palindromic repeats. It describes patterns observed in bacterial DNA that code for RNA guide molecules that target specific DNA sequences. These guide molecules pair with a CRISPR-associated protein (Cas), which cuts the DNA when the guide RNA . These bacterial molecules have been harnessed by scientists to create CRISPR-based genome editing technology.
The most common type of CRISPR, CRISPR/CAS9, consists of a guide RNA modified to target a particular DNA sequence plus Cas9, which cuts through both strands of the DNA once it finds its target. Once created, this break in the DNA allows for DNA to be added or removed at the target site; conveniently, the cell’s own repair mechanisms will fix the breaks.
Base editing uses a CRISPR guide RNA to bind its target sequence, but instead of cutting the DNA strand, it chemically changes one DNA base letter into another. So, base editing is part of the wider family of CRISPR genome editing approaches, but it does not work in the ‘cut and paste’ way most associated with CRISPR/Cas9.
- Find out more: CRISPR therapeutics (a LinkAGE webinar)
What is base editing used for?
Base editing is best suited to making single-nucleotide changes to a DNA sequence. Because it chemically changes the target letter, it can only make one change at a time. This has advantages and disadvantages.
CRISPR/Cas9 can make much larger changes: it can remove or insert pieces of DNA into the target strand. However, to do this it makes double-stranded breaks in the DNA, which carries the risk of errors being introduced. These errors can include cuts in the wrong part of the genome (off-target effects) and unintended indels (where fragments of DNA are lost or gained when the break is repaired).
Applications in healthcare
Base editing has the potential to positively impact healthcare because it carries a lower risk of creating indels, and because single-nucleotide changes are associated with many genetic diseases in humans, including sickle cell disease and Tay-Sachs disease.
In a recent article, we described how base editing was used to make four single-nucleotide changes to the genome of blood stem cells in order to create a CAR-T cell therapy for a young girl with otherwise untreatable leukaemia.
When making CAR-T cells, the genome editing takes place outside the body, so there is an opportunity to check for errors before the cells are given to the patient. However, base editing is being looked at as a possible tool for in-body gene editing: as part of a major study into cardiomyopathy, researchers are hoping to develop gene therapies using base editing. This is because many inherited cardiomyopathies result from single-nucleotide changes.
In July 2022, a familial hypercholesterolaemia patient in New Zealand became the first to receive an in-body base-editing gene therapy as part of a clinical trial aiming to permanently deactivate the PCSK9 gene in liver cells. In some people, PCSK9 causes high levels of low-density lipoprotein cholesterol, which, over time, causes arteries to clog and can result in heart attacks and strokes. The therapy, known as VERVE-101, was created by biotechnology company Verve Therapeutics; co-founder and CEO Dr Sekar Kathiresan said of the findings, “Preclinical data suggest that VERVE-101 has the potential to offer people [with familial hypercholesterolemia] a game-changing treatment option, transforming the traditional chronic care model to a single-course, life-long treatment solution.”
–