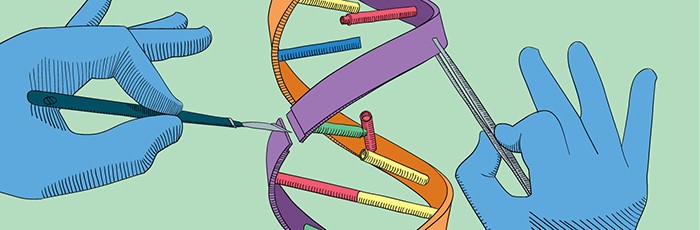
Casgevy – the first CRISPR therapy
The UK has approved a new type of gene therapy for the blood disorders sickle cell disease and beta-thalassaemia, a world-first using CRISPR technology
The Medicines and Healthcare products Regulatory Agency (MHRA) has approved Casgevy, a gene therapy treatment for sickle cell disease and transfusion-dependent β-thalassaemia. Approximately 17,500 people in the UK have sickle cell disease. Both of these disorders are genetic conditions that affect an important protein called haemoglobin, which is found in red blood cells and is essential for carrying oxygen around the body.
“Both sickle cell disease and β-thalassemia are painful, life-long conditions that in some cases can be fatal. To date, a bone marrow transplant – which must come from a closely matched donor and carries a risk of rejection – has been the only permanent treatment option,” said MHRA Interim Executive Director of Healthcare Quality and Access Julian Beach, when the decision was announced.
- Further reading: What are genome editing and gene therapy?
CRISPR-based therapy
Casgevy is a gene therapy that utilises the innovative Nobel Prize-winning gene-editing tool CRISPR. The process works by taking the patient’s bone marrow stem cells and editing them to express the fetal version of haemoglobin before transplanting these edited stem cells back into the patient.
Fetal haemoglobin is the version expressed in utero, before birth. In most people, expression of the fetal version is turned down and the non-fetal version is turned on when they are a baby, although there is still usually a very small amount of fetal haemoglobin expressed. Casgevy edits this by turning up the expression of the fetal version; the functional haemoglobin produced from this version of the gene compensates for the non-functional haemoglobin that the non-fetal version of the gene is producing.
The edited stem cells are the patient’s own cells, just slightly edited, and as such there is no risk of rejection. The results have the potential to be life-long.
- Further reading: CRISPR: From ‘silly idea’ to miracle cures?
Approval of Casgevy
The MRHA’s approval of Casgevy (also known as Exa-cel) makes the UK the first country to approve a CRISPR-based medicine. The decision was made after results from clinical trials to assess the safety and efficacy of the treatment were carefully considered, and the MHRA will continue to monitor these factors as the treatment begins to be used after authorisation.
“Trials [have] been found to restore healthy haemoglobin production in the majority of participants with sickle-cell disease and transfusion-dependent β -thalassaemia, relieving the symptoms of disease”, said Julian Beach.
Although the UK is the first to approve the drug, the US may not be far behind. The Food and Drug Administration (FDA) is expected to decide whether or not to approve Casgevy in the United States in the next few weeks, after it was favourably assessed by The Cellular, Tissue, and Gene Therapies Advisory Committee in October 2023.
Will Casgevy be available on the NHS?
The short answer is ‘not necessarily’.
The MRHA decision means that Casgevy can be sold in the UK, and doctors here can legally prescribe it to patients aged 12 or over with sickle cell disease, or transfusion-dependent β-thalassaemia. However, the decision about whether it should be funded for NHS patients will be made separately by the National Institutes of Clinical Excellence (NICE).
According to NICE’s website, guidance on the therapy’s use for β-thalassaemia patients is expected in March 2024, with the decision for its application in sickle cell disease to follow in April 2024. NICE’s decision will consider the evidence for the effectiveness of the treatment, alone and compared with existing treatments. It will also consider the cost of Casgevy, which has not yet been made public but is expected to be over $1 million USD.
This would be in line with pricing for other single-dose ex-vivo gene therapies that have been approved: for example, Zolgensma for spinal muscular atrophy is priced at just under £1.8 million, although the NHS negotiated a confidential discount from the manufacturer.