Introduction
Recent advances in genomics mean that increasing numbers of children and their families can benefit from genomic testing. Testing can provide new diagnoses, shape management decisions, guide treatment options and provide valuable information. Paediatricians need a broad understanding of genomics to ensure that their patients have access to genomic tests at the right time.
In this film, consultant neonatologist Dr Ngozi Edi-Osagie talks about why genomics matters in paediatrics, how it can be applied in a paediatrician’s day-to-day clinical practice, and how paediatricians can play a part in the development of genomic medicine across the NHS.
What do I need to know?
Why is genomics important in paediatrics?
Many genomic conditions affect early development, leading to congenital structural malformations and/or neurodevelopmental delay. This means that individuals with genomic conditions will commonly present in childhood.
By identifying a diagnosis, you can help patients and their families in a wide variety of ways. Some examples are outlined below.
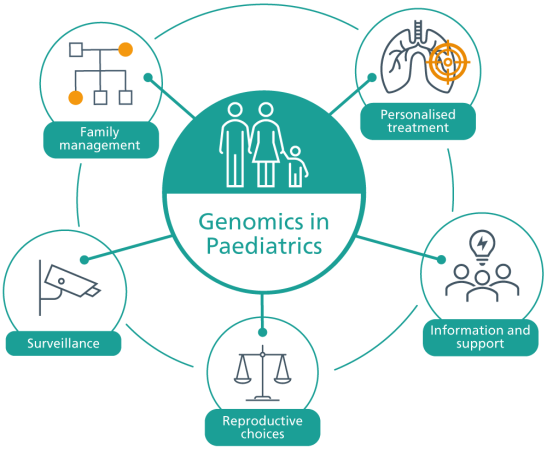
Information and support
Having a genomic diagnosis often allows the patient and their family – as well as other healthcare professionals – to learn more about their condition, understand the health implications in the short and long term, and access family support groups, which may be helpful from both an emotional and an educational perspective. Support groups often inform their members about developments in the field, including research and clinical trials.
Watch this short video about Beskida and Arvin’s experience of receiving a genomic diagnosis.
Surveillance
Some paediatric genomic conditions are associated with specific health complications, such as an increased risk of developing early childhood tumours. For some of these complications, specific surveillance guidelines have been developed and published; this means that if the genomic condition is diagnosed early on, the child will have timely access to a condition-specific surveillance programme that could improve their healthcare outcomes by identifying and enabling management of complications (which may include personalised treatments) at an early stage.
Personalised treatment
In some cases, a genomic diagnosis provides access to personalised treatment. Some examples of this are outlined below.
- The use of commonly-used drugs proven to be particularly effective in a specific genomic condition. For example, the anticonvulsant retigabine (ezogabine) has particular efficacy in treating epilepsy caused by loss-of-function variants in the KCNQ2 gene.
- The avoidance of certain medications. For example, some babies (1 in 500) are born with a particular genetic variant that means that administering the antibiotic gentamicin will cause irreversible hearing loss; however, new technologies have enabled clinicians to test for this genetic variant with a single cheek swab, which has a 15-minute turnaround time for results and means that use of gentamicin can be avoided where necessary.
- The use of targeted therapies – many of which are in development – such as enzyme replacements, dietary restrictions or gene therapies. For example, patients with spinal muscular atrophy are now benefiting from treatment with nusinersen (widely marketed as Spinraza), a drug that is delivered directly to the central nervous system to target an underlying cause of motor neuron degeneration. Other similar treatments are also becoming more widely available in the UK.
Family management and reproductive choices
Because we share some of our DNA with people to whom we are genetically related, genomic conditions can have implications for our family members. This means that we must consider the inheritance pattern of any genomic diagnosis. It may be appropriate to offer parental testing, which helps to clarify any inheritance patterns and potential risks to immediate family members. For some conditions, it may be appropriate to offer cascade testing to the wider family.
Where there is a significant chance of future pregnancies being affected by the same genomic condition, some couples may wish to make complex, personal reproductive choices that may include a normal pregnancy (sometimes with additional scans or screening), use of donated sperm, egg and/or embryo, adoption, prenatal diagnosis or pre-implantation genetic diagnosis (PGD). Prenatal diagnosis is usually performed through chorionic villus sampling between 11 and 14 weeks of pregnancy. PGD is an IVF-based technique in which embryos are screened and only those without the familial genomic variant are transferred into the womb. In these scenarios, your role as a paediatrician would be to refer your patients to the appropriate specialist team – be it genetic counselling, fetal and women’s health or clinical genetics.
Finally, children with a genomic condition may be anxious about and want to discuss the implications of their diagnosis for any future children they themselves may have. An understanding of the inheritance patterns of genomic conditions will help you, as a paediatrician, to have those conversations with affected children.
What happens when genomic tests are inconclusive?
Many families will not receive a diagnosis through initial genomic testing. Trying to find the genomic variant responsible for a condition or syndrome is difficult, and sometimes it’s not possible: we are still limited by technology and our current knowledge base. When requesting genomic testing for a child, there are some important things for clinicians to know and do to help maximise the chance that a diagnosis will be identified. You can find out more about this in our dedicated resource.
Bear in mind that a normal genomic test does not rule out a genomic cause. Where a diagnosis is not identified, it is important to consider whether any further genomic testing would be beneficial. In cases in which no further genomic testing is currently available, it is important to remind families that it may be available in the future. It is also important to support families who do not receive a diagnosis by, for example, signposting them to patient support groups such as SWAN UK, a network run by Genetic Alliance UK. You can find out more about how to support families without a diagnosis in our rare disease education hub.
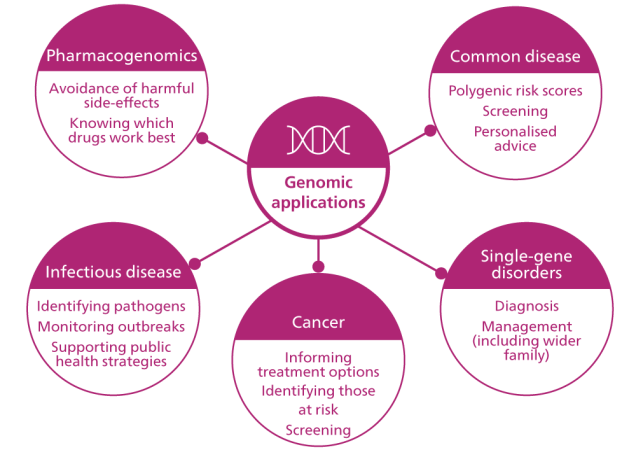
How can genomics be applied in paediatrics?
The number of ways in which genomics can be applied in paediatrics will only increase with time. Take a look at the diagram below to find out some of the ways in which genomics is already being used to improve paediatric patient outcomes.
What is my role as a paediatrician?
Below are just a few examples of the ways in which paediatricians can play a part in the development of genomic medicine across the NHS.
Facilitating genomic testing
Many paediatricians have been ordering genomic tests and managing results for years. For others, genomic testing may be completely new. Whatever your background, now is a good time to develop your skills in this area because the landscape of genomics is changing rapidly. For example, the National Genomic Test Directory is currently expanding the number of genomic tests available, and increasing your understanding of the latest genomic technologies and available tests can help you identify more patients who could benefit from testing.
Seeking informed consent
Paediatricians are increasingly ordering more complex genomic tests. It is therefore important to have a good understanding of the potential implications of testing, so that informed consent can be sought. This should be documented as part of a record of discussion.
Understanding a genomic test report
Once you have requested a genomic test and it has been carried out, the results will be analysed by clinical scientists in the genomic laboratory and a report will be produced. The responsibility for interpreting the implications of the report and communicating them to the patient and their family will sit with you, as the clinician who requested the test – though support will be available from clinical genetics specialists, and there may be local multi-disciplinary team (MDT) meetings at which you can discuss the results. To learn more about reading, understanding and communicating genomic test reports, watch our video tutorial.
While some genomic test reports may be relatively simple, others can be more complex. For example, a variant of uncertain significance may have been identified – that is, a variant for which there is insufficient data to determine whether it is causative of or contributing towards the patient’s clinical presentation. Rarely, an incidental or secondary finding may be identified that describes a genomic variant that is causative of disease (for example, a cancer predisposition syndrome), but not of the patient’s clinical presentation. Most variants of uncertain significance require a referral to a clinical genetics team.
Watch this video to learn more about variants of uncertain significance.
Managing a genomic diagnosis
Genomic testing has become more important in the acute management of some patients. The availability of broader and more rapid tests means that rare diagnoses can be identified in a timely manner, often with significant management implications.
Genomic discoveries are also being made in children with chronic and complex conditions, providing answers for families and healthcare professionals and clarifying the need for surveillance or referrals to the wider MDT.
As genomics becomes more mainstream, paediatricians will be taking more of a leadership role in the continuity of care of children with genomic conditions.
Working with genomics specialists
Paediatricians and clinical geneticists work closely in the management of rare genomic conditions in children. A referral to clinical genetics is indicated in many cases: for instance, cases in which a genomic diagnosis is suspected but testing has not identified one, or where further information and family testing for a new genomic diagnosis is required. In addition, MDTs that include clinical geneticists and paediatricians are increasingly being established; these MDTs provide a forum to discuss management of a genomic diagnosis, the interpretation of a genomic result or the appropriateness of a potential referral.
Supporting research
All the advances in genomics that we are now witnessing result from research. Research continues to support these advances, and will result in new genomic applications that improve the lives of children. Paediatricians can support their patients to take part in research by, for example, giving patients who are undergoing whole genome sequencing the option to submit their anonymised data to the National Genomic Research Library.
Case example: Bardet-Biedl syndrome
- Antenatally, George was found to have an echogenic kidney (a kidney that appears bright in an ultrasound, indicating a possible condition). He was also born with an extra fifth digit (a condition known as polydactyly), and was consequently referred to the clinical genetics team.
- When George was reviewed by the clinical genetics team as a baby, results of microarray testing and a postnatal renal ultrasound scan were both normal.
- George was followed up by the clinical genetics team until the age of two. There were no further suspicions of a genetic disorder, so he was discharged back to paediatric care, where he was later managed for developing obesity and mild learning difficulties.
- Due to evolving genomic technologies, George was then recruited into a research study into early-onset obesity by his paediatrician.
- Two years later, the research study found two variants in George’s BBS5 gene, indicating a diagnosis of autosomal recessive Bardet-Biedl syndrome (BBS).
- As well as presenting a cause for George’s polydactyly, obesity and learning difficulties, this new diagnosis had important implications for his management and prognosis. Sadly, most individuals with BBS become blind by their second or third decade of life.
- George was referred back to the clinical genetics team for further support and counselling about the condition, while his paediatrician made clinical referrals to the ophthalmology, audiology, endocrinology and renal teams for surveillance of his syndromic manifestations.
In the Clinic: GeNotes Paediatrics
Try GeNotes (genomic notes for clinicians), our scenario-based resource, providing quick, concise information to support you when ordering genomic tests and returning back results.
Learning opportunities
Teaching aids
Clinical resources
Identifying patients
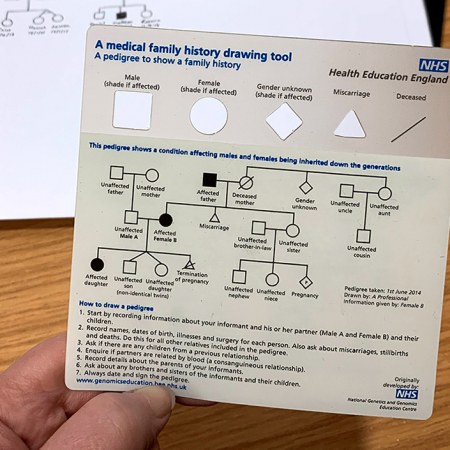
Activity: Taking and drawing a genetic family history
Equip yourself with the knowledge and skills to construct a genetic pedigree.
Activity: Recognising the clinical clues of a genetic condition
Familiarise yourself with the clinical features of a range of genetic conditions
Communicating with patients
Activity: Returning genomic test results to patients
There are a range of factors to consider when returning genomic results to patients, based on whether there is a confirmed diagnosis, an uncertain result or a negative result.