Introduction
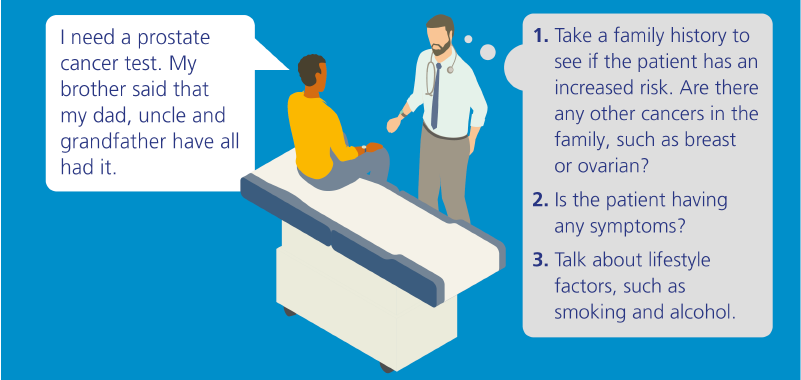
There are dramatic advances being made in the field of genomic medicine and these increasingly have an impact in primary care. As the gateway to the NHS, primary care practitioners are vital to the early identification of genomics issues and to ensuring appropriate management and quality of care – often throughout a person’s lifetime.
Watch this film to hear Dr Jude Hayward explain the increasing importance of genomics in primary care and the role practitioners play in the delivery of personalised medicine.