Statins
Some individuals have genetic variants in the SLCO1B1, CYP2C9 and ABCG2 genes, which can increase the risk of statin-induced myopathy.
Clinical context
Statins are used for the primary and secondary prevention of cardiovascular disease and are among the most commonly prescribed medicines. Statins can cause a wide range of muscle-related adverse events, including myalgia, myopathy and statin-induced rhabdomyolysis, known collectively as statin-associated musculoskeletal symptoms (SAMS).
Statin therapy and pharmacogenomics
- Statins lower cholesterol production by inhibiting the 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase enzyme.
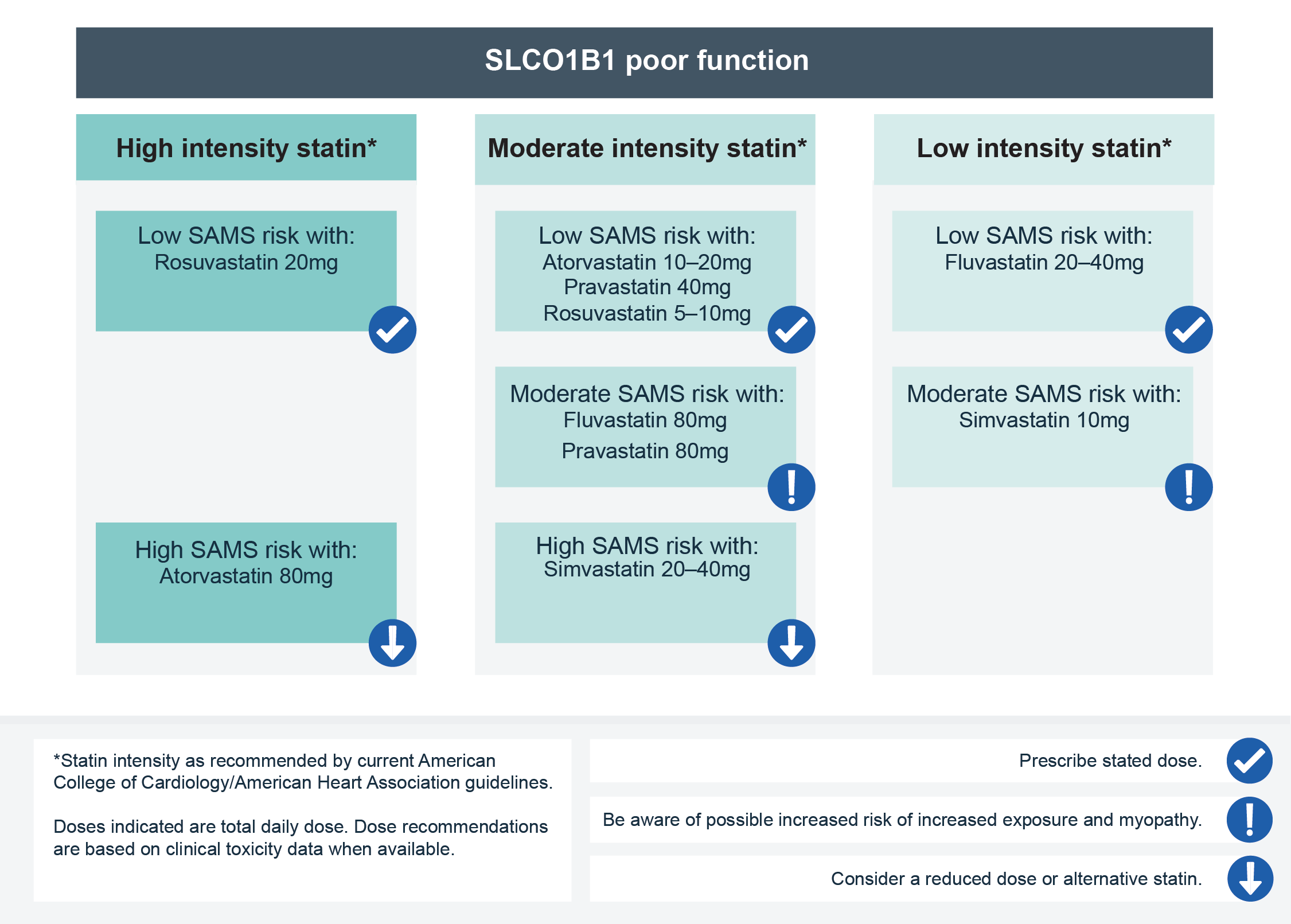
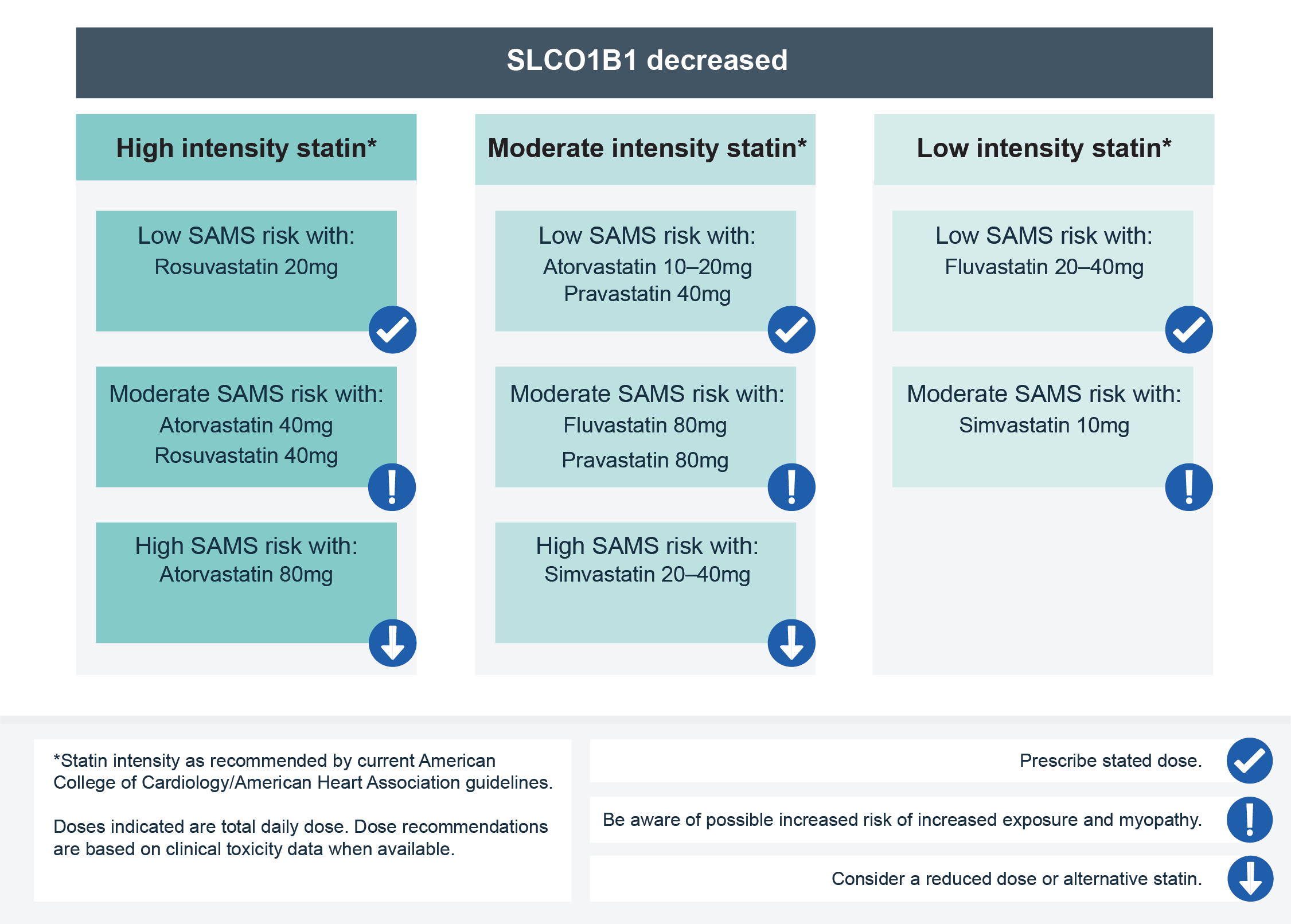
- They are transported into the liver by the OATP1B1 transporter protein, which is encoded by the gene SLCO1B1. Genetic variation in SLCO1B1 can reduce its activity, resulting in increased systemic exposure to most statins and an increased risk of SAMS. In particular, the evidence that links the common SLCO1B1 genetic variant (rs4149056, also known as c.521T>C or Val174Ala) to myopathy is strongest for simvastatin and considered moderate for atorvastatin.
- CYP2C9 is a hepatic cytochrome P450 enzyme that is associated with the metabolism of fluvastatin, but does not affect other statins. Reduced CYP2C9 activity, caused by genetic variation in the CYP2C9 gene, increases systemic exposure to fluvastatin, which can predispose an individual to increased risk of fluvastatin muscle adverse events.
- The efflux transporter, ABCG2, is involved in the efflux of rosuvastatin. Individuals with poor ABCG2 function, caused by genetic variation in ABCG2, have increased systemic exposure to rosuvastatin. It is currently considered uncertain as to whether this translates clinically into an increased risk of myopathy on rosuvastatin.
Genomic testing for SLCO1B1, CYP2C9 and ABCG2
- Testing for SLCO1B1, CYP2C9 and ABCG2 is not currently available via the National Genomic Test Directory.
- Patients may present with pharmacogenomic information from other healthcare systems, clinical trials or, increasingly, from direct-to-consumer genomic testing (caution should be exercised when interpreting results from non-validated genomic tests).
- Both the Clinical Pharmacogenetics Implementation Consortium (CPIC) and the Dutch Pharmacogenetics Working Group (DPWG) have produced pharmacogenomic prescribing recommendations for statins (see the resources list below).
- For more information, see Results: Patient with known SLCO1B1 genotype requiring statin therapy.
Resources
For clinicians
- Electronic medicines compendium
- NHS England: National Genomic Test Directory
- PharmGKB: Annotation of CPIC guideline for atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, simvastatin and ABCG2
- PharmGKB: Annotation of CPIC guideline for fluvastatin and CYP2C9, SLCO1B1
- PharmGKB: Annotation of CPIC guideline for simvastatin and SLCO1B1
References:
- Cooper-DeHoff RM, Niemi M, Ramsey L and others. ‘The Clinical Pharmacogenetics Implementation Consortium guideline for SLCO1B1, ABCG2 and CYP2C9 genotypes and statin-associated musculoskeletal symptoms’. Clinical Pharmacology and Therapeutics 2022: volume 111, issue 5, pages 1,007–1,021. DOI: 10.1002/cpt.2557