Multiple endocrine neoplasia type 1
Multiple endocrine neoplasia type 1 is a condition associated with variants in the MEN1 gene that causes predisposition to tumours in the endocrine organs.
Overview
Multiple endocrine neoplasia type 1 (MEN1) is a condition that causes predisposition to tumours in the endocrine organs. It is associated with constitutional (germline) pathogenic variants in the MEN1 gene.
Clinical features
Clinical features of MEN1 include:
- parathyroid tumours or parathyroid hyperplasia;
- pituitary tumours (most often prolactinomas, followed by non-functioning pituitary tumours, acromegaly and, rarely, adrenocorticotropic hormone (ACTH)-secreting or thyroid stimulating hormone (TSH)-secreting tumours);
- pancreatic neuroendocrine tumours;
- other endocrine tumours (such as endocrine tumours of the gastro-entero-pancreatic tract and/or adrenocortical tumours); and
- other non-endocrine tumours (such as facial angiofibromas, collagenoma and/or meningioma) – see figure 1.
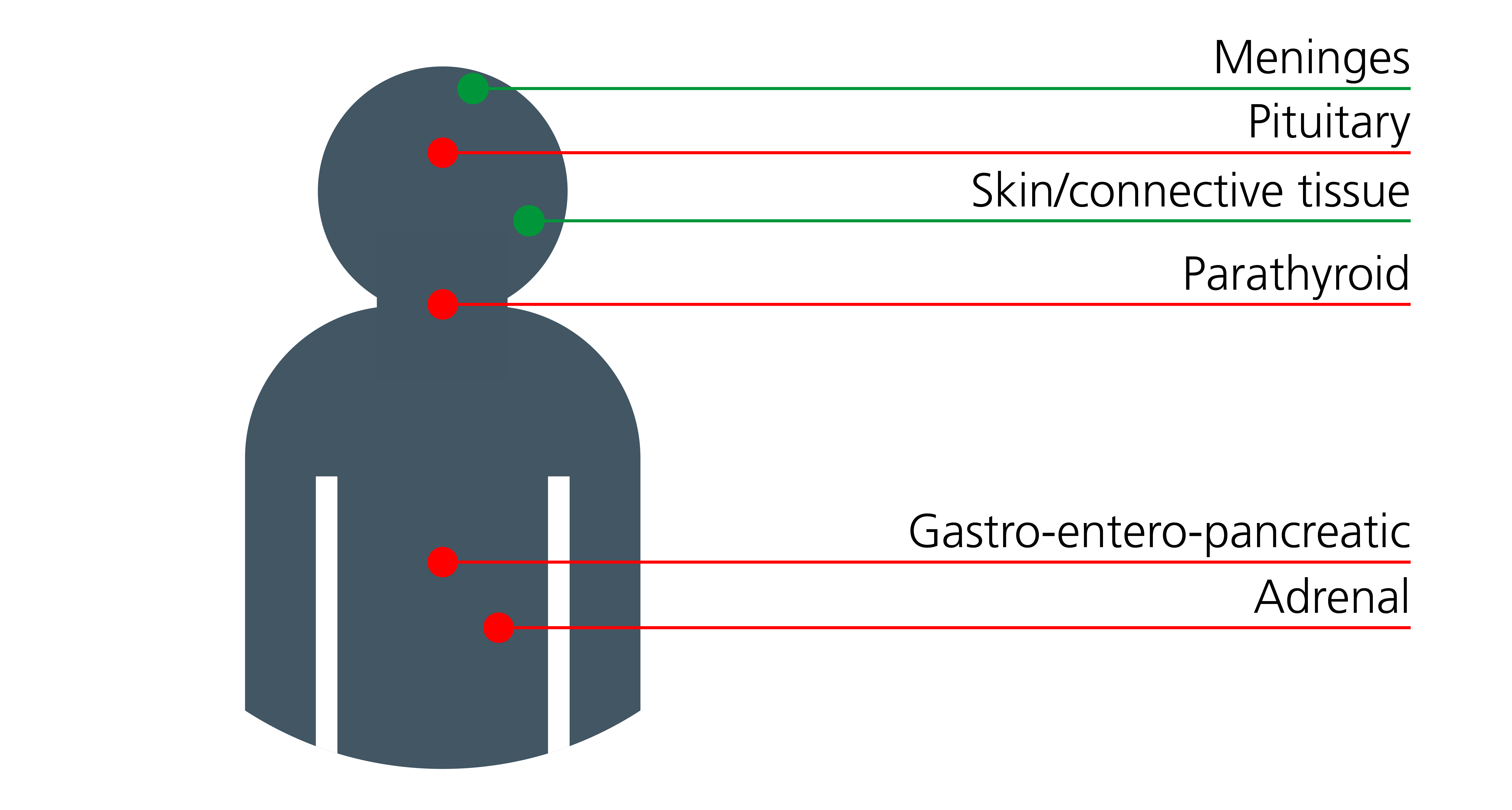
Figure 1: Sites of endocrine and non-endocrine tumours associated with MEN1
Note: Endocrine tumours are shown in red; non-endocrine tumours are shown in green. Skin and connective tissue tumours may include collagenomas and angiofibromas.
Genomics
MEN1 is caused by constitutional (germline) pathogenic loss-of-function variants in the MEN1 tumour suppressor gene, which codes for the menin protein.
Menin can interact with many other proteins and is thought to regulate gene transcription via chromatin remodelling. Interacting proteins include the cell cycle regulators p27 and p18, which may explain the phenotypic similarity with multiple endocrine neoplasia type 4 (which is caused by constitutional (germline) pathogenic variants in CDKN1B, the gene coding p27).
The majority of pathogenic MEN1 variants result in a truncated protein. To date, there is no clear genotype-phenotype correlation in MEN1. It can be caused by de novo variants in MEN1 (around 10% of cases) and, rarely, it can take a mosaic form. MEN1-like syndromes may occur in patients without MEN1 gene alteration.
For information about genomic testing, see Presentation: Patient with endocrine neoplasia and Presentation: Patient with possible multiple endocrine neoplasia type 1.
Diagnosis
The diagnosis of MEN1 hinges on clinicians having a high degree of clinical alertness in patients presenting with different components of the syndrome or patients presenting with a component of the syndrome and a relevant family history. A clinical diagnosis of MEN1 can be made in:
- an individual with two or more MEN1-related endocrine abnormalities at any age;
- an individual with one or more MEN1-related endocrine abnormalities and one or more MEN1-related non-endocrine tumours at any age; or
- an individual with one or more MEN1-related endocrine abnormalities and a first-degree relative with one or more MEN1-related endocrine abnormalities.
Individuals presenting with parathyroid multiglandular disease (hyperplasia/adenomas) under the age of 35 years, a pituitary adenoma or insulinoma under the age of 20 years, or a pituitary macroadenoma under 30 years should be offered genetic testing for MEN1, regardless of the presence of other manifestations or family history.
Inheritance and genomic counselling
MEN1 is an autosomal dominant condition. It is almost fully penetrant by 40 years of age in at least one of the manifestations. Individuals affected by an autosomal dominant condition have one working copy of the gene, and one with a pathogenic variant. A parent with a pathogenic variant has a 1 in 2 (50%) chance of passing the variant on to their child.
Family planning implications
The Human Fertilisation & Embryology Authority has approved the use of preimplantation genetic testing for monogenic disorders (PGT-M) (previously known as pre-implantation genetic diagnosis) for couples in whom one potential parent is a carrier of a pathogenic variant in MEN1. It is best practice that discussions regarding PGT-M and other family planning options be undertaken by a specialist genetic counsellor or a clinical geneticist.
Alternative options may include:
- prenatal testing (invasive, or non-invasive if the intended father is the carrier) with termination of affected embryos;
- adoption;
- gamete donation; or
- natural conception and pregnancy with testing of children at an appropriate age (current MEN1 guidelines (see resources section below) advocate for clinical review of children with MEN1 pathogenic variants from around the age of five).
Management
Management and screening of individuals with MEN1 should follow published guidelines (see table 1 and the resources section below), with screening and surveillance starting in childhood. Endocrine neoplasia syndromes are multisystem conditions and care is best delivered by a multidisciplinary team.
Table 1: Screening in individuals at high risk of developing MEN1
| Tumour | Age to begin screening (years) | Annual biochemical test (plasma or serum) | Imaging test (time interval) |
| Parathyroid | Eight | Calcium, parathyroid hormone (PTH) | None |
| Gastrinoma | 20 | Gastrin (with or without gastric pH) | None |
| Insulinoma | Five | Fasting glucose, insulin | None |
| Other pancreatic neuroendocrine tumours | Under 10 | Chromogranin-A, pancreatic polypeptide, glucagon, VIP | MRI (preferably in children), CT or EUS (annually) |
| Anterior pituitary | Five | Prolactin, insulin-like growth factor 1 (IGF-1) | MRI (every three years) |
| Adrenal | Under 10 | None, unless symptoms or signs of functioning tumour and/or tumour under 1cm are identified on imaging | MRI (preferably in children) or CT (annually; simultaneous with pancreatic imaging) |
| Thymic and bronchial carcinoid | 15 | None | MRI (preferably in children) or CT (every one to two years) |
Resources
For clinicians
- Genomics England: Genomic Medicine Service (GMS) Signed Off Panels Resource
- NHS England: National Genomic Test Directory
References:
- Brandi ML, Pieterman C, English K and others. ‘Multiple endocrine neoplasia type 1 (MEN1): recommendations and guidelines for best practice’. The Lancet Diabetes & Endocrinology 2025: volume 13, issue 8, pages 699–721. DOI: 10.1016/S2213-8587(25)00119-6
For patients
- Association for Multiple Endocrine Neoplasia (AMEND)
- AMEND: Starting a family: Your choices (online booklet, 34 pages)
- Parathyroid UK
- Neuroendocrine Cancer UK
- The Pituitary Foundation